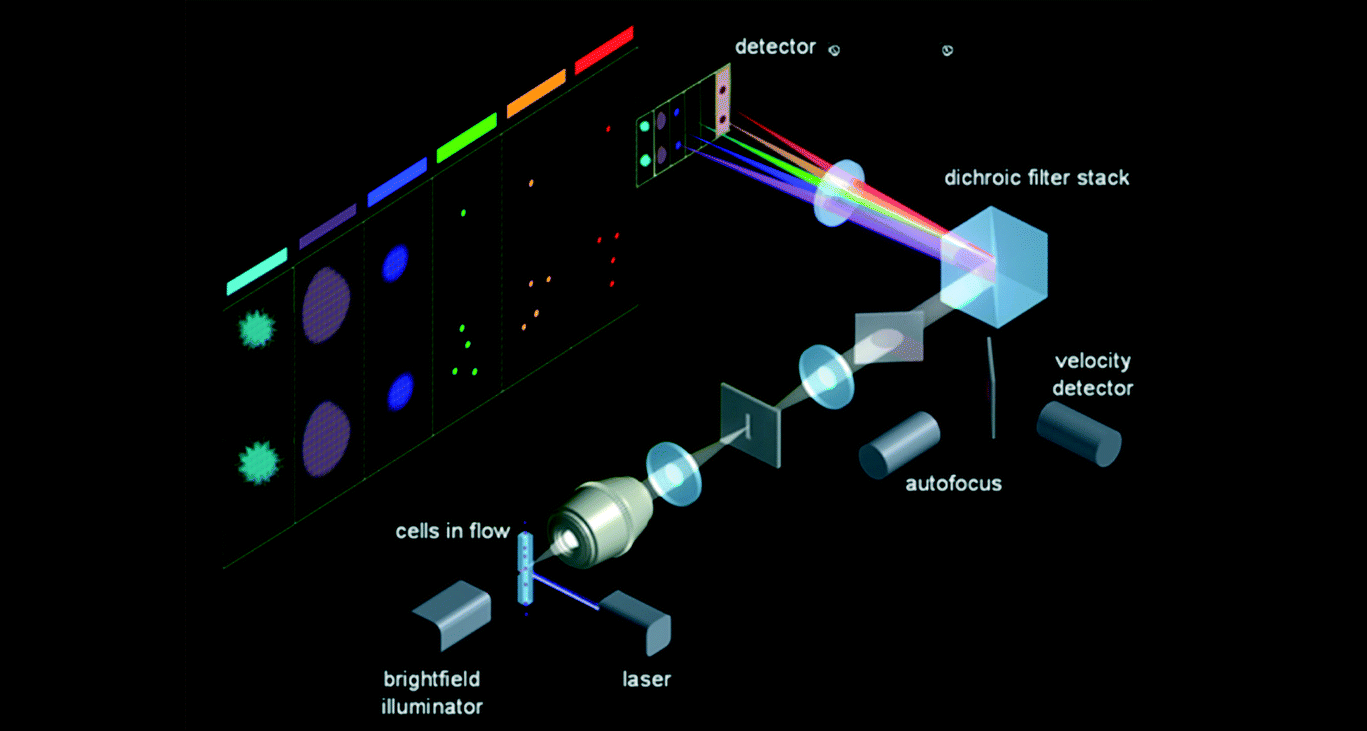
Flow cytometry is a method for simultaneously analyzing multiple physical properties of an individual cell as it flows through a beam of light in a fluid stream, including the cells size and fluorescence. In practice, flow cytometry is essentially a combination of particle counting and fluorescence spectroscopy. For more on the fundamentals of these two technologies we recommend you check out our previous blog post on Optical Particle Counting and our white paper on Multi-Color Fluorescence. By combining these two techniques flow cytometry can be employed in cell counting, cell sorting, biomarker detection, and protein engineering analyzing thousands of cells per second as they pass through the liquid stream. In this blog post, we will explore the differences between traditional particle counting instrumentation and those used in flow cytometry. Furthermore, we will discuss how these differences affect the choice of laser or multiple lasers for the particular application, and how this adds additional complexity to the system. It is important to note that this blog post is not meant to serve as an overview of the applications of flow cytometry but rather the fundamental practicalities required for successful laser selection and implementation.
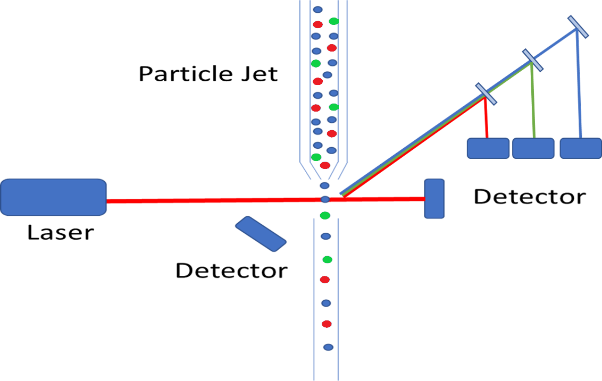
On a fundamental level, the basic instrumentation set-up is very similar to that of traditional particle counting devices, where a particle jet is used to direct a stream of individual particles through the laser path. Where the complexity arises is that we are no longer merely measuring the intensity of scattered light, but we are also required to measure the wavelength of the fluorescence emission. As we discussed in our previous white paper on the subject, fluorophores can be functionalized and bound to living cells. This provides the ability to identify particular cells which fluorophores of varying emission bands, to sort cells based on a wide variety of criteria. Typically, these bands are relatively broad and fairly spread out from each other eliminating the need for costly spectrometers, but the emission path must still be filtered, and dichroicly separated. This allows for each individual emission band to be detected using a single (or double for redundancy purposes) photodetector. The figure below shows a schematic representation of how a particle counter could be modified to measure fluorescence from tagged cells. In this diagram, the different cells are color-coded red, green, and blue, and the fluorescence emission is collected off axis to minimize excitation laser bleed-through.
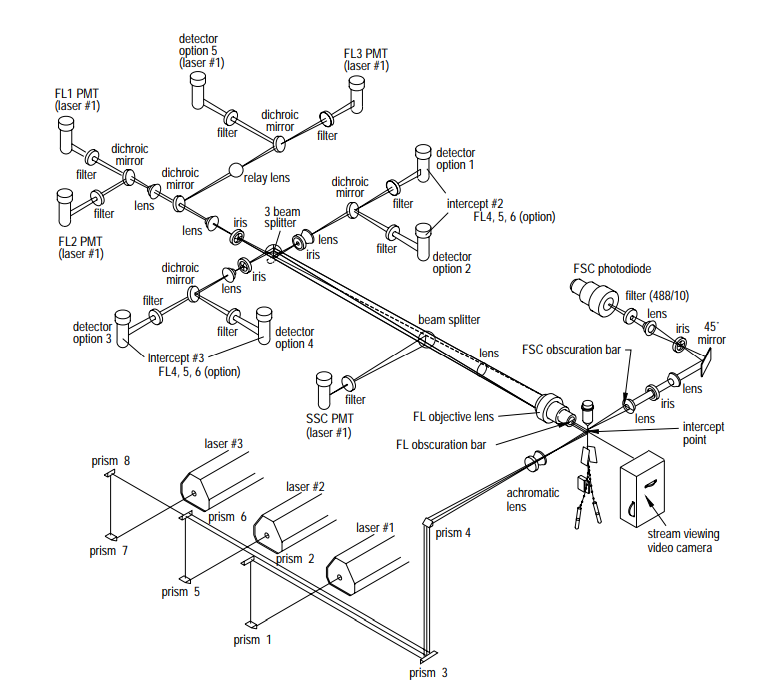
In the example above, only a single excitation laser source is used, but this limits the number of potential fluorophores that the end user can choose. As was also discussed in our recent white paper on Multi-Color Fluorescence, by selecting several laser lines spaced throughout the visible spectral range, one should be able to excite a wide variety of fluorophores. This exponentially increases the list of potential fluorophores and therefore, the specific cell properties that can be probed. Below is an example of an actual flow cytometer from Becton, Dickinson, and Company’s Biosciences division the BD FACSVantage SE™. This schematic, which was taken from Becton, Dickinson, and Company’s Introduction to Flow Cytometry: A Learning Guide, shows how the system uses three different excitation wavelengths and four collection wavelengths. This system allows for over 50 different combinations of excitation and emission bands, giving the user incredible flexibility in fluorophore selection.
There are many different flow cytometry based applications. With multiple wavelengths at your disposal, allowing such diverse combinations as mentioned above, all of these applications are made more accessible. Some of these applications include cell sorting, immune cell phenotyping (immunophenotyping), immune cell function analysis, intracellular cytokine staining analysis, receptor occupancy analysis, gene therapy, cell cycle analysis, cell proliferation, membrane potential, live/dead bacteria discrimination, tumor suppressor gene/protein expression, antigen-specific cell responses, and many others.
Just as in traditional particle counting, these lasers must exhibit excellent pointing and power stability, and single-mode, low noise operation (typically free-space output). However, unlike conventional particle counting systems, the wavelengths must be chosen to match the excitation spectra of the available fluorophores. Typical wavelengths include 355nm, 405nm, 473nm, 488nm, 532nm, 553nm, 561nm, 594nm, 640nm and NIR, with output powers in the 25-500mW range. Additionally, since multiple lasers are being integrated into a single system, size, cost, and ease of integration all become significant factors in deciding which laser to choose. Here at RPMC lasers, we offer a unique ultra-compact laser source which is capable of providing a low noise (0.4% typical) single-mode (typical M2 of 1.3) output beam with laser housing dimensions of only 50 mm x 30 mm x 18 mm. These lasers are available from 405 nm to 1064 nm and are capable of producing output powers as high as 500mW.
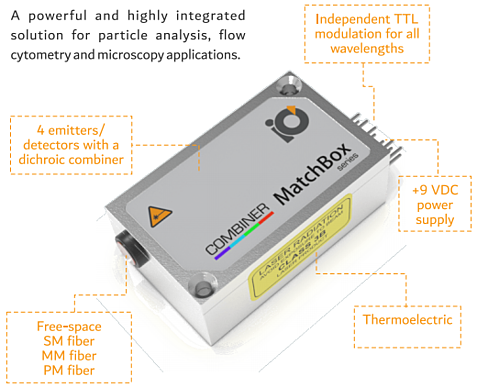 To support flow cytometry, we also offer the MatchBox Multi-Wavelength Combiner. This multi-wavelength laser diode combiner series is a configurable, ultra-compact, turn-key, multicolor laser system. It utilizes a classical dichroic combining technique to combine four channels for laser diodes or photodetectors into a single optical path. The package includes two to four laser diode drivers, TEC driving electronics, a microprocessor, and the precision-aligned electro-optical part, all inside the world’s smallest footprint of 30x50x18 mm. As a result, the laser/detector unit provides unprecedented compactness and functionality. The unit is designed as an integration-ready electro-optics unit, which can be connected to a control mainboard and power supply of an instrument, perfect for OEM and portable/handheld devices and applications. Check out this previous blog: “World’s Smallest 4-Wavelength Combiner Gets Some BIG Upgrades!“
To support flow cytometry, we also offer the MatchBox Multi-Wavelength Combiner. This multi-wavelength laser diode combiner series is a configurable, ultra-compact, turn-key, multicolor laser system. It utilizes a classical dichroic combining technique to combine four channels for laser diodes or photodetectors into a single optical path. The package includes two to four laser diode drivers, TEC driving electronics, a microprocessor, and the precision-aligned electro-optical part, all inside the world’s smallest footprint of 30x50x18 mm. As a result, the laser/detector unit provides unprecedented compactness and functionality. The unit is designed as an integration-ready electro-optics unit, which can be connected to a control mainboard and power supply of an instrument, perfect for OEM and portable/handheld devices and applications. Check out this previous blog: “World’s Smallest 4-Wavelength Combiner Gets Some BIG Upgrades!“
The Matchbox series offers excellent performance and reliability in an ultra-compact “all-in-one” integrated laser head. They come standard with an integrated internal voltage up-conversion that allows using a 5V power supply while maintaining low noise operation. The monolithic design of the Matchbox Series laser includes thermally stabilized optics in a hermetically sealed housing, ensuring reliable and maintenance-free operation. All the Matchbox series modules include a 12-month warranty and are RoHS compliant. All of these features make them the ideal laser source for integration into commercial flow cytometers.
For detailed technical specifications on the full range of ultra-compact single-mode lasers, click here or talk to one of our laser experts today by calling 1-636-272-7227 or by contacting us Here.

 SHIPS TODAY
SHIPS TODAY 