Introduction
Fluorescence spectroscopy is a powerful technique for identifying the presence of particular molecular species of interest. This technique has allowed for many of the advances in biochemistry that we now take for granted today. For example, fluorescence spectroscopy is the cornerstone of DNA sequencing for the identification of the four basic DNA molecules, adenine (A), cytosine (C), guanine (G), and thymine (T) by tagging them with fluorescence reporter molecules. A detailed analysis of fluorescence tagging will be discussed later in the article, but for now, it’s important to understand that this allows for the rapid determination of the precise order of A, C, G, and T molecules, so the entire DNA strand to be coded [1]. Another typical application of fluorescence spectroscopy comes from the field of anti-counterfeiting where fluorescent tags are added to the ink of currency, which when illuminated by the proper excitation wavelength emit a unique fluorescence spectrum known only to the manufacturer [2]. In this particular article, we are going to mainly focus our attention on the application of fluorescence in the field of microscopy, where it can be used to generate multispectral images of cells and other small objects for identifying the spatial distribution of molecules of interest in complex heterogeneous samples.
Theory of Fluorescence Spectroscopy
Fluorescence is the process by which a molecule absorbs a photon whose energy is significantly large enough to excite the system from the ground state (So) to a higher electronic energy level (S1). Once the system is in the excited state, it wants to relax back down to the more stable ground state, but in many cases, the nonradiative decay time between the vibrational bands of the excited state is much shorter than the radiative decay time between the two electronic energy levels. As a result, some energy is lost in the form of heat via this non-radiative decay, resulting in the emission of a photon with less energy than the absorbed photon due to the conservation of energy. Therefore, the emitted photon is shifted to a longer wavelength compared to the absorbed photon, this shift in wavelength is commonly referred to as a Stokes shift and is demonstrated in figure 1 below.
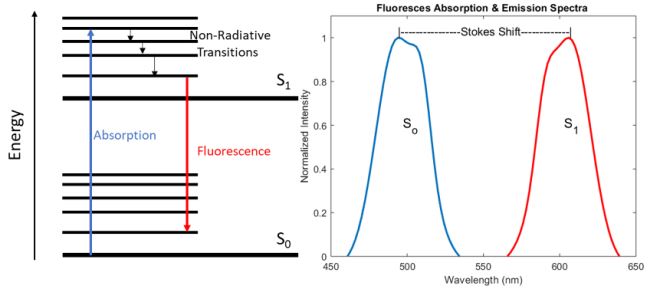
While an exhaustive analysis of the chemical structures that lead to the absorption and emission of specific wavelengths of light is beyond the scope of this article, it is important to note that the vibrational mode structure and the bandgap between S0 and S1 are highly dependent on the bond structure, particularly the presence of double bonds which utilize less tightly bound -electrons. As a result, molecules known as fluorophores can be engineered to have specific absorption and emission properties which can be tailored for a particular application, for a more detailed analysis, please refer to the first chapter in the Handbook of Fluorescence Spectroscopy and Imaging: From Ensemble to Single Molecules by Sauer et al. 2011 [3].
Furthermore, these fluorophores can be functionalized to bond with specific molecules of interest creating chemically sensitive fluorescence tags such as the ones discussed previously for DNA sequencing.
Fluorescence Microscopy
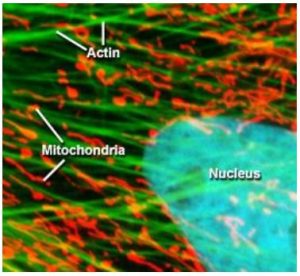
Otto Heimstaedt and Heinrich Lehmann, where the first to realize the possibility of fluorescence microscopy while experimenting with ultraviolet microscopes between 1911 and 1913. While their initial intention was to increase the spatial resolution of the microscope by using shorter wavelength light, they quickly realized that ultraviolet excitation was inducing autofluorescence in their samples [4]. However, these first instruments suffered from one major drawback, the high energy ultraviolet light would cause photochemical damage to the sample, especially living cells. Fortunately, in 1914 Stanislav Von Prowazek showed for the first time that fluorophores could be functionalized and bound to living cells [4] allowing the excitation and emission wavelengths to be engineered independently of the sample’s natural properties. Therefore, fluorescent tags quickly became standard practice in fluorescence microscopy allowing the excitation energy required to induce fluorescence to be significantly reduced. In turn, this decreased photochemical degradation of the sample by moving from ultraviolet to visible excitation wavelengths, which was extremely advantageous for biological studies allowing for sample integrity to be maintained especially for live samples. As a result, fluorescence microscopy has become one of the most widely utilized techniques in biological sciences. Figure 2 below shows an example of a cell where the three fluorophores where functionalized to attached themselves to the actin, mitochondria, and the nucleus [5].

 SHIPS TODAY
SHIPS TODAY 